Normalization of both VWF and FVIII to ensure normal hemostasis1
Avoidance of FVIII accumulation with repeated dosing of VWF/FVIII, which may increase risk of thrombosis5
Close monitoring of VWF and FVIII levels to avoid under- or over-dosing6
Prophylaxis with VWF complex*
Normalization of both VWF and FVIII to ensure normal hemostasis1
Avoidance of FVIII accumulation with repeated dosing of VWF/FVIII, which may increase risk of thrombosis5
Close monitoring of VWF and FVIII levels to avoid under- or over-dosing6
Parallel decay of VWF:RCo and FVIII:C
May simplify dosing and monitoring
WONDERS: Prospective, open-label, uncontrolled Phase III study of wilate® in surgery
FVIII: factor VIII
Overall efficacy rated as successful* in 97% of surgeries
Success in major surgeries (20/21)
Success in minor surgeries (9/9)
Success in 21 surgeries in 20 patients with type 3 von Willebrand disease
*IDMC-adjudicated treatment success rating based on haemostatic efficacy assessment (intraoperatively by surgeon and postoperatively by investigator) using objective 4-point scale (excellent, good, moderate, none). FVIII: factor VIII
Loading Dose
Maintenance Dose

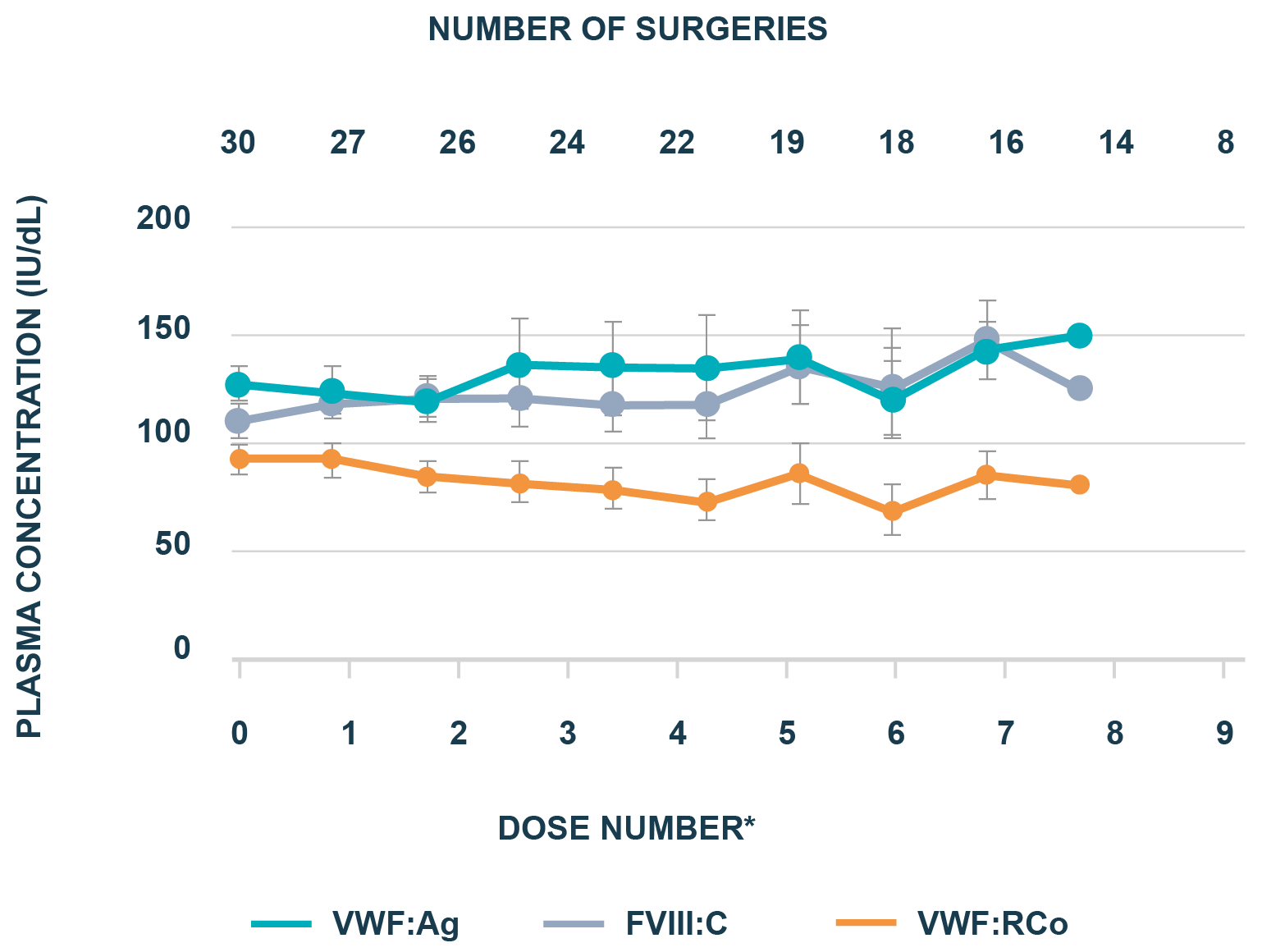
WIL-20: Prospective, observational Phase IV study on the use of wilate® in von Willebrand disease
*Diagnosis data not available
WIL-20: Prospective, observational Phase IV study on the use of wilate® in von Willebrand disease
of 46 major surgeries,
of 52 minor surgeries,
of all surgeries
*IDMC-adjudicated treatment success rating based on haemostatic efficacy assessment (intraoperatively by surgeon and postoperatively by investigator) using objective 4-point scale (excellent, good, moderate, none).
Median dose of wilate® per procedure
For Major Surgeries
Range: (22–500 IU/kg)
For Minor Surgeries
Range: (6–680 IU/kg)
Reported in the
WIL-20 study
*Hemostatic effectiveness assessment after surgery using objective 4-point scale (excellent, good, moderate, none). Effectiveness rating not available for one minor surgery.
Leebeek FWG and Eikenboom JCJ. N Engl J Med 2016; 375:2067–80.
Miesbach W and Berntorp E. Eur J Haematol 2017; 98:121–27.
Srivastava A et al. Haemophilia 2017; 23:264–72.
Windyga J and von Depka-Prondzinski M. Thromb Haemost 2011; 105:1072–9.
Mannucci PM. N Engl J Med 2004; 351:683–94.
Kessler CM et al. Thromb Haemost 2011; 106:279–88.
Sholzberg M et al. TH Open 2021; 5:e264–72.
Batty P et al. Haemophilia 2014; 20:846–53.
Khair K et al. Haemophilia 2015; 21:e44–50.
Please see wilate® full Prescribing Information.
Indication
wilate® is a von Willebrand Factor/Coagulation Factor VIII Complex (Human) indicated in children and adults with von Willebrand disease for on-demand treatment and control of bleeding episodes; for perioperative management of bleeding; and for routine prophylaxis to reduce the frequency of bleeding episodes in children 6 years of age and older and adults with VWD. wilate® is also indicated in adolescents and adults with hemophilia A for routine prophylaxis to reduce the frequency of bleeding episodes; and for on-demand treatment and control of bleeding episodes.
Contraindications
wilate® is contraindicated in patients with known hypersensitivity reactions, including anaphylactic or severe systemic reactions, to human plasma-derived products, any ingredient in the formulation, or components of the container.
Warnings and Precautions
Hypersensitivity Reactions
Hypersensitivity reactions may occur with wilate®. Signs and symptoms include angioedema, burning and stinging at the infusion site, chills, flushing, generalized urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, and wheezing that may progress to severe anaphylaxis (including shock) with or without fever. Closely monitor patients receiving wilate® and observe for any symptoms throughout the infusion period.
Because inhibitor antibodies may occur concomitantly with anaphylactic reactions, evaluate patients experiencing an anaphylactic reaction for the presence of inhibitors.
Thromboembolic Events
In VWD, continued treatment using a FVIII-containing VWF product may cause an excessive rise in FVIII activity, which may increase the risk of thromboembolic events. Monitor plasma levels of VWF:RCo and FVIII activities in patients receiving wilate® to avoid sustained excessive VWF and FVIII activity levels.
Neutralizing Antibodies
VWD
Hemophilia A
Transmissible Infectious Agents
wilate® is made from human plasma. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the variant Creutzfeldt-Jakob disease (vCJD) agent. There is also the possibility that unknown infectious agents may be present in the product. The risk that wilate® will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, it may still potentially transmit disease.
Record the batch number of the product every time wilate® is administered to a patient, and consider appropriate vaccination (against hepatitis A and B virus) of patients in regular/repeated receipt of wilate®. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Octapharma USA, Inc., at 1-866-766-4860.
Monitoring and Laboratory Tests
Adverse Reactions
The most common adverse reactions to treatment with wilate® (≥ 1%) in patients with VWD were hypersensitivity reactions, urticaria, chest discomfort and dizziness. The most common adverse reactions to treatment with wilate® (≥ 1%) in previously treated patients with hemophilia A was pyrexia (fever).
Please see wilate® full Prescribing Information.
Please see wilate® full Prescribing Information.
Indication
wilate® is a von Willebrand Factor/Coagulation Factor VIII Complex (Human) indicated in children and adults with von Willebrand disease for on-demand treatment and control of bleeding episodes; for perioperative management of bleeding; and for routine prophylaxis to reduce the frequency of bleeding episodes in children 6 years of age and older and adults with VWD. wilate® is also indicated in adolescents and adults with hemophilia A for routine prophylaxis to reduce the frequency of bleeding episodes; and for on-demand treatment and control of bleeding episodes.
Contraindications
wilate® is contraindicated in patients with known hypersensitivity reactions, including anaphylactic or severe systemic reactions, to human plasma-derived products, any ingredient in the formulation, or components of the container.
Warnings and Precautions
Hypersensitivity Reactions
Hypersensitivity reactions may occur with wilate®. Signs and symptoms include angioedema, burning and stinging at the infusion site, chills, flushing, generalized urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, and wheezing that may progress to severe anaphylaxis (including shock) with or without fever. Closely monitor patients receiving wilate® and observe for any symptoms throughout the infusion period.
Because inhibitor antibodies may occur concomitantly with anaphylactic reactions, evaluate patients experiencing an anaphylactic reaction for the presence of inhibitors.
Thromboembolic Events
In VWD, continued treatment using a FVIII-containing VWF product may cause an excessive rise in FVIII activity, which may increase the risk of thromboembolic events. Monitor plasma levels of VWF:RCo and FVIII activities in patients receiving wilate® to avoid sustained excessive VWF and FVIII activity levels.
Neutralizing Antibodies
VWD
Hemophilia A
Transmissible Infectious Agents
wilate® is made from human plasma. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the variant Creutzfeldt-Jakob disease (vCJD) agent. There is also the possibility that unknown infectious agents may be present in the product. The risk that wilate® will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, it may still potentially transmit disease.
Record the batch number of the product every time wilate® is administered to a patient, and consider appropriate vaccination (against hepatitis A and B virus) of patients in regular/repeated receipt of wilate®. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Octapharma USA, Inc., at 1-866-766-4860.
Monitoring and Laboratory Tests
Adverse Reactions
The most common adverse reactions to treatment with wilate® (≥ 1%) in patients with VWD were hypersensitivity reactions, urticaria, chest discomfort and dizziness. The most common adverse reactions to treatment with wilate® (≥ 1%) in previously treated patients with hemophilia A was pyrexia (fever).
Please see wilate® full Prescribing Information.
Octapharma does not review, endorse, or control the content of any non-Octapharma site. Octapharma is not responsible for the accuracy, content, practices, or standards of any non-Octapharma resources accessed through a link on this site. Octapharma provides general information for educational purposes. This site does not provide medical advice, diagnosis, or treatment to you. Always consult your physician to help decide a treatment plan or other medical advice.
The information on this website has been specifically created for U.S. healthcare professionals (HCPs)
Please see wilate® full Prescribing Information