Safety and Effectiveness of wilate for von Willebrand Disease Treatment
wilate has been proven to be safe and effective
for the treatment of bleeding in VWD
The safety and effectiveness of wilate was tested in 4 different clinical studies. These studies included adult and pediatric patients with mild, moderate, and severe VWD, and included more than 1,000 bleeding episodes.
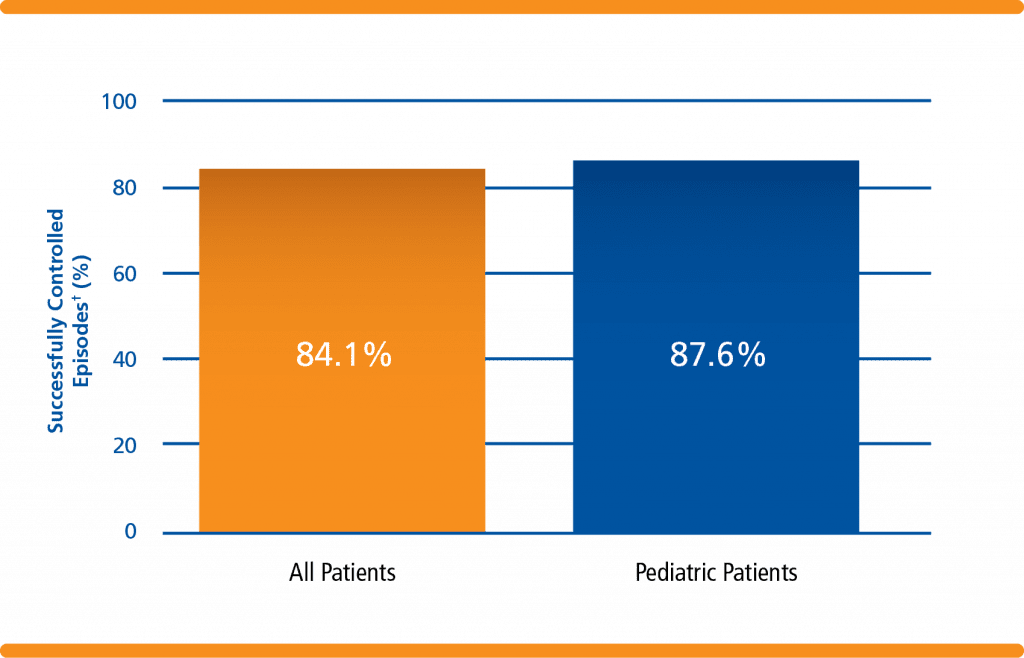
Successful treatment of bleeding episodes in patients with VWD1,*+
*A “bleeding episode” may involve bleeding at multiple sites in this analysis. The most common bleeding sites were joints, nose bleeds, stomach bleeds, and mouth bleeds.
†Among 70 VWD patients, 45 patients received wilate on-demand treatment for 1,068 bleeding episodes, including 11 pediatric patients (5-16 years of age), treated for 234 bleeding episodes.1
- For all patients studied, 84% of bleeding treatments were successful
- For pediatric patients studied, 88% of bleeding treatments were successful
- 93% of the successfully treated bleeds occurred in type 3 patients, the most severe type of VWD
wilate is proven to be safe and effective in minor
and major surgery2
Success in prevention and treatment of bleeding
By type of surgery
| Efficacy assessment* | % Success |
|---|---|
| Minor (N-9) | 100% (9/9) |
| Major (N-21) | 95.2% (20/21) |
| All surgeries (N-30) | 96.7% (29/30) |
*Based on the derived overall assessment (derived from the intra- and postoperative assessment of the IDMC, based on blood loss, transfusion requirements, and postoperative bleeding and oozing). IDMC, Independent Data Monitoring Committee.
†Two patients were enrolled for 2 surgeries each; therefore, there were 28 individual patients
- Efficacy was excellent or good in all surgical procedures with children (3 surgeries in 3 children)
- There were no thromboembolic events
wilate safety and tolerability
In clinical trials, 92 VWD patients received 5,676 infusions of wilate 1
- There were no reported cases of thrombotic events1
- The most common adverse reactions were hypersensitivity (allergic) reactions, urticaria (hives),
and dizziness (each in 2.2% of patients) - The most serious adverse reactions were hypersensitivity reactions.
1 Billion
Since 2005, more than 1 billion units of wilate have been infused worldwide.3Reference:
- wilate Full Prescribing Information. Paramus, NJ: Octapharma; rev December 2023.
- Srivastava A, et al. Haemophilia. 2017;23(2):264-272.
- Data on file. Paramus, NJ: Octapharma USA Inc. 2018